NK Therapeutics
For a long time, research has been carried out on immune cell therapy to treat cancer by administering immune cells such as NK cells and T cells after in vitro culture, but no clear therapeutic effect has been achieved. A more effective cell therapy has been developed by introducing CAR (Chimeric Antigen Receptor) into T cells using gene editing technology. CAR-T cell therapy showed a dramatically improved therapeutic effect in some blood cancer patients, and in 2017, the US FDA designated CAR-T cell therapy as an innovative treatment and approved commercialization.
Since then, CAR-T cell therapy products have received worldwide attention, and numerous companies and research institutes have tried to develop CAR-T cell therapy products with improved therapeutic efficacy by using various gene editing technologies. However, CAR-T cell therapies in some cases cause serious life-threatening side effects, such as CRS (Cytokine Release Syndrome) and Neurotoxicity. Moreover, it has yet to prove its efficacy against solid cancers, which affect many more patients. Accordingly, there is an increasing need for new CAR-NK cell therapies that can replace CAR-T cells.
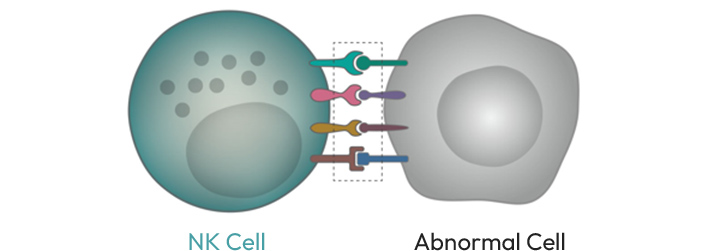
NK cells are a type of innate immune cell that not only protects the human body from various pathogens that invade from the outside, but also help the body to maintain homeostasis by selectively removing abnormal cells from the body via immune surveillance, a process controlled by various cell surface receptors. It is an optimal cell for introducing CAR because there is a significantly lower possibility of side effects such as CRS and Neurotoxicity. NK cells can precisely discriminate healthy cells from cancer cells and specifically remove only cancer cells.
Therabest has been researching for a long time to maximize the therapeutic effect while retaining the advantages of NK cells, and has developed EiNK™ platform technology.
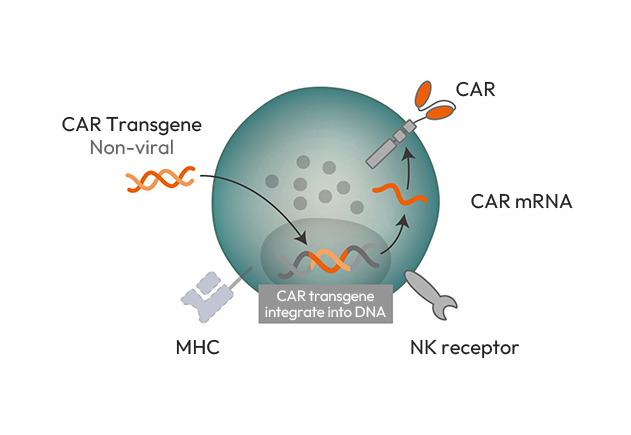
EiNK™ (Enhanced iPSC-derived NK cell) is an allogeneic CAR-NK cell therapy platform in which non-viral gene editing technology is used in iPSCs prior to NK cell differentiation.
EiNK™ will be an innovative next-generation immune cell therapy that can combat solid cancers.
-
- Strong therapeutic effect
- EiNK™ precisely removes cancer cells expressing specific cancer antigens through CAR, and at the same time, they can even remove cancer cells that do not express cancer antigens by utilizing their other immune surveillance receptors of NK cells. In addition, Armored technology to maximize the effect of NK cells was introduced together with CAR, which was designed to further strengthen therapeutic effect.
-
- Safety
- Since EiNK™ uses non-viral gene editing technology, there is no risk of residual virus. In addition, the possibility of genetic mutation is very low because the position and number of genes inserted are known and verified. In addition, EiNK™ can be safely used for many patients because factors that can cause GvHD and immune rejection are removed by introducing Universal technology.
-
- Uniformity
-
Using conventional CAR introduction technologies, such as viral delivery systems, the average CAR expression rate of immune cells is 20 to 60%, meaning the expression is not uniform.
EiNK™ with Theabest's iPSC technology has a very high average CAR expression rate of over 95%, so a higher anti-tumor treatment effect can be expected.
-
- Productivity
-
Since EiNK™ is mass produced from iPSCs, there is no material limit to EiNK™ production, and donor-dependent batch-to-batch variation is eliminated.
Using gene-editing technology in master iPSC lines can further enhance the universal application of EiNK™ as an off-the-shelf allogeneic cell therapy.